Main page >> Moulds and their characteristics >> How moulds are classified >> Where moulds are found >> How moulds can be isolated >> How moulds are grown >> Contamination >> Moulds under the microscope >> How moulds are identified >> Index of descriptions >> Bibliography
MOULDS UNDER THE MICROSCOPE
There are many good texts on the theory and use of the microscope and I am thus going to assume the reader either has some knowledge of microscopy or can find it. My main interest here is with the particular skills necessary for microscopic examination of moulds.
Preparation of slides
Most beginners find moulds difficult to prepare for microscopic examination. Often preparations seem to contain only spores or mycelium, or structures that are so unlike any of the illustrations available that they are unidentifiable. Most of these problems can be overcome with a little practice and will, in time, seem trivial.
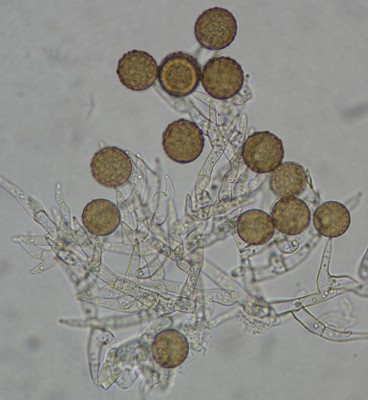
The first rule to remember in mould studies is to examine young, actively growing material. Older parts of colonies or moulds on natural material will often be partially decomposed or so covered with spores as to be unrecognizable. The best way to begin is to examine the growth from the margin of the colony where spores are being actively produced. It may require two or three attempts before the area of active sporulation is located, but when it is all the features of spore production necessary for identification can be seen. If the colony has been in Petri plate with other moulds for several weeks and is no longer actively spreading it will not yield good material for examination.
To make a good microscopic preparation from a mould colony, start by putting a small drop of mounting medium on a microscope slide. Microscope mounting media come in a variety of types and will be discussed separately; at the beginning, use water. Then, using a sterilized dissecting or inoculating needle remove a small (no more than 2 mm square) portion of the colony near the margin, taking with it a very thin layer of the agar surface. If the colony is thick and woolly, it may not be necessary to take the agar, but in the more appressed type it is essential. Place the piece of colony in the mounting medium, and, with a second needle, tease it out so that the filaments are well spread. A mount that has not been teased out will appear as an opaque lump yielding little information. Place a cover-slip over the mount, lowering one edge to the slide before the other so that air bubbles can escape. The remaining air bubbles can be removed from the mount by gently heating it over an alcohol flame. If it is heated too vigorously, the cover-slip will pop off explosively, splattering fungus and medium into one's face, so it is essential to heat it only until its steams slightly, not until it boils. The picture of Harzia verrucosa above was made from a simple water mount and photographed through the eyepiece of a microscope using a point-and-shoot digital camera. This is usually all that is needed to see what you want.
An interesting technique used by some mycologists for preparing microscope mounts involves sticking the mould to a bit of cellophane tape. The tape is pressed lightly against the colony so that some of the hyphae and spores stick to it. This is then placed sticky side up in a drop of mounting fluid (see below) on a microscope slide and covered with a cover slip. This technique is not restricted to colonies in Petri dishes; it works well on naturally occurring colonies in most habitats. The cellophane tape technique is commonly used for sampling moulds in indoor environments.
Slide cultures
Most moulds will yield good results if prepared as outlined above, but a few present extra difficulties. The most problematic are those that tend to disintegrate as soon as they are mounted. Species of Cladosporium, Monilia, and Alternaria have spores connected in very fragile chains that can fall apart at the slightest movement of air. Mounts of these fungi invariably reveal only loose spores and a network of hyphae. To overcome this problem it is useful to set up slide cultures (figure 13). Slide cultures are made by setting up a small Petri dish moist chamber containing a V-shaped piece of glass tubing resting on several layers of moistened filter-paper. A sterile block of agar medium about 1 cm square is placed on a flame-sterilized microscope slide and the slide is then set in the moist chamber on the tubing. The fungus is inoculated near the four edges of the agar block and a sterile cover-slip is put over it. After a few days the slide can be mounted on a microscope, and the undisturbed mould structures viewed as they are growing. Later, if desired, the agar block can be removed from slide and cover-slip and two conventional slide mounts made from them. If they are allowed to dry before this is done, the mould structures are less likely to break apart.
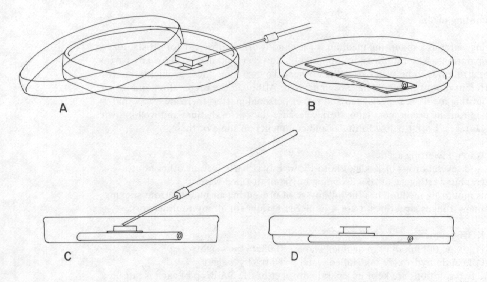
Figure 13. Slide culture technique. A block of sterile agar is cut out of a Petri dish (A) and is placed upon a sterile slide resting on a bent glass tube within a sterile Petri dish (B). A few spores of a fungus are inoculated at the edges of the sterile agar block (C) and topped with a cover-glass (D) for incubation. A disc of moist filter-paper in the dish maintains humidity for the culture.
Mounting media and stains for microscopy
Using water as a mounting medium is easy and is often sufficient for satisfactory preparations. Water mounts dry up quickly, however, and do not allow any particular structures to be seen better than others. To overcome these problems, many different mounting media have been devised. Although the preparation and use of mounting media is a specialized and rather personal matter, there are a few that are in routine use in most laboratories because they offer distinct and well-known advantages. I offer below formulas and comments on some of these.
| Congo Red Solution | |
|---|---|
| Congo Red | 1.0 g | Distilled water | 100 ml |

The Congo red crystals may not dissolve completely in the water, so you should pour the solution through a filter to render it free of solids.
The dye Congo Red has an affinity for the cell walls of fungi while not staining the cell contents very much. Because it is used in a very dilute solution with water it causes almost no distortion of fungal tissues. This gentle mounting medium is particularly prized for demonstrating delicate structures such as clamp connections on basidia and croziers on asci.
Congo Red is not used most effectively in its original solution because it is dark red and doesn't allow stained material to be viewed with maximum contrast. Thick mounts are generally not very useful in Congo Red. The best way to use it is to immerse the sample in a drop of the Congo Red, wait 5 to 10 minutes and then transfer it to a drop of water. The specimen can then be transferred to yet another drop of water where it appear red against a colourless background. Then apply a cover slip and examine it with the microscope. Sometimes fungal material does not revive very well in water and it is useful to use a drop of 5% KOH solution for the final drop. One warning here: Congo Red is a strong dye, even at high dilutions, and can stain fingers and clothing if not handled carefully. The photo at right is of a basidium of a Gymnopilus species (a mushroom) mounted in Congo Red Solution.
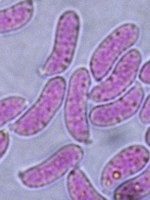
| Phloxine Solution | |
|---|---|
| Solution 1 | |
| Phloxine ( a pink dye) | 0.025 g |
| Distilled water | 100 ml |
This is a useful staining medium for basidiomycetes and other fungi with compact and difficult-to-spread tissues. The mould hyphae are stained a bright pink colour and are thus more easily seen than in water mounts. It is best used in the same manner described above for Congo Red. That is, stain the material in the Phloxine solution and then wash out the excess dye by transferring through successive drops of water. As with Congo Red, the final drop can be 5% KOH. The picture at right is of basidiospores of Dacrymyces lacrymalis mounted in KOH + phloxine.
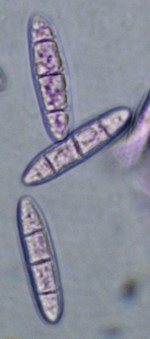
| Lacto-fuchsin | |
|---|---|
| Acid fuchsin (a pink dye) | 0.1 g |
| Lactic acid (pure) | 100 ml |
Lacto-fuchsin will not dry up on the slide for several weeks and is thus useful if a slide must be saved for awhile. Drying can be prolonged even further by sealing the edges of the cover-slip with clear nail polish. Lacto-fuchsin is a strong stain that is especially useful for mounts of anamorphs and other easily spread structures.
| Melzer's Solution | |
|---|---|
| Chloral hydrate | 100 g |
| Potassium iodide | 5.0 g |
| Iodine | 1.5 g |
| Distilled water | 100 ml |
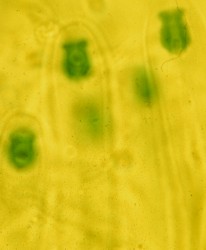
This mounting medium is used extensively in mycology. Certain tissues turn blue to blackish in it and are said to be amyloid; others stain red or dark orange and are called dextrinoid. In the illustration at right the asci of Ustulina vulgaris were treated with Melzer's Solution. The ordinarily clear ascal plugs have stained blue. Melzer's Solution is a good general mounting medium that clears the material somewhat and allows particularly brilliant resolution with a microscope. For good resolution and colour in photographs through the microscope, I make up Melzer's Solution without the iodine, which yields a very clear solution. WARNING: Chloral hydrate should be handled with care as it is toxic. In addition it may be listed as a controlled substance in some jurisdictions, requiring special permission for purchase and possession.
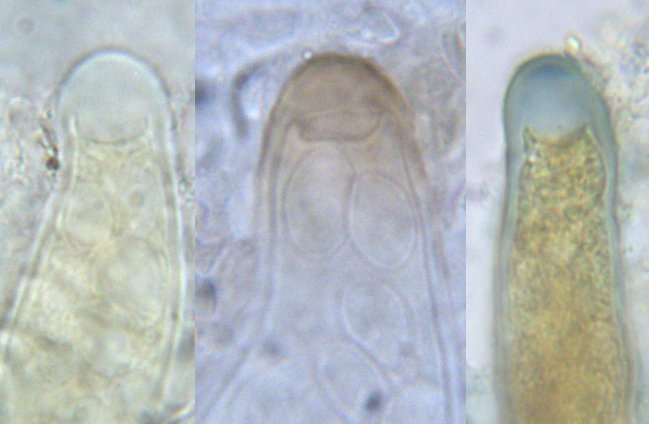
Some mycologists prefer to use iodine-containing mounting media without chloral hydrate. Lugol's Solution (0.5 g iodine, 1.5 g potassium iodide, 100 ml distilled water) and IKI (1.0 g iodine, 3.0 g potassium iodide, 100 ml distilled water) are both substitutes for Melzer's Solution and will produce an amyloid or dextrinoid reaction in many fungi. In fact lichenologists (people who study lichens) routinely use Lugol's and only rarely use Melzer's. The amount of iodine can be varied according to the sensitivity of the group of fungi being studied. Although arguments have been made in favour of all of these media, using them in combination will probably yield the most information. The photo at right shows three asci of Agyrium rufum, a fungus commonly encountered on dry branches in sunny locations. The one at left was mounted in Melzer's Solution and yielded no colour changes. The one in the middle was placed in heated 5% KOH solution and then transferred to Melzer's, giving a brown reaction to the ascus tip (perhaps due more to the KOH than to the Melzer's). The one at right was mounted in Lugol's Solution and shows an amyloid apex and dextrinoid contents. This illustration seems to indicate that Lugol's Solution is the best treatment to use, but it's not that simple: many structures can be strongly amyloid in Melzer's alone, as seen in the picture of Ustulina vulgaris, or in Melzer's with KOH pretreatment, so only using all three techniques will give a complete profile.
| Shear's mounting medium | |
|---|---|
| Potassium acetate | 6 g |
| Glycerine | 120 ml |
| Ethanol 95% | 180 ml |
| Distilled water | 300 ml |
| Ink Blue | 1 g |
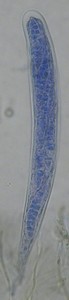
A very good, all-purpose mounting medium that does not dry upon the slide for several weeks. As with Lacto-fuchsin, it can be sealed in with nail polish for even longer life. Ink Blue was not a part of the original formula but is useful as a stain for the walls of certain fungi. The picture at right shows an ascus of a Claussenomyces species mounted in Shear's Mounting Medium with Ink Blue. The ascospores and ascus contents have become stained blue while the walls of the ascus remain unstained.
| Water + wetting agent | |
|---|---|
| Distilled water | 100 ml |
| Wetting agent | A few drops |
Several products intended to be used as photographic wetting agents, such as Kodak Photo-Flow, can be used, but with the rapid disappearance of photographic film these products may be hard to find. Polysorbate 20, sold commercially under such brand names as Alkest TW 20 and Tween 20) are available from scientific supply houses. Detergent may also work. Try dish detergent, using no more than 5 drops per liter of water. If you use too much you will get soap bubbles
More recently we have begun using Windex, a widely available product for cleaning windows, as a convenient mounting medium. It contains a small amount to ammonium hydroxide, plus some surfactants, and is quite useful for quick mounts. Material placed in Windex rehydrate rapidly and appear remarkably fresh.
These mounting media have the advantage of preventing air bubbles from sticking to many fungus structures. I sometimes use them in place of water for making mounts of particularly dry moulds. Many fungi are difficult to "wet", even when a wetting agent is used. For these, I suggest putting them in a drop of 95% ethyl alcohol for a few seconds and then, before the alcohol is completely dried out, adding a drop of the required mounting medium. This often works wonders with the dryest of moulds.
Certain mounting media, such as lacto-phenol, are widely used in mycological laboratories but are very toxic and offer no particular advantages over those listed above.