Main page >> Moulds and their characteristics >> How moulds are classified >> Where moulds are found >> How moulds can be isolated >> How moulds are grown >> Contamination >> Moulds under the microscope >> How moulds are identified >> Index of descriptions >> Bibliography
HOW MOULDS CAN BE ISOLATED
One of the joys of mycology is being able to go out into the field and isolate from among thousands of moulds a single interesting species. Almost any natural substance will have not one mould but a whole community of moulds growing on it. Each mould is distinct from all others and produces a characteristic colony when grown in pure culture. The isolation, or we might say domestication, of each is a challenge that never seems to lose its fascination.
A most difficult thing for the beginner is to come to terms with the small scale of mould communities. Even an area only 1 millimetre square can support more than one species of mould when nutrients are available. The only really practical way to get down to this scale is to work with a binocular dissecting microscope of sufficient magnification to allow spore-bearing structures to be seen. When this is possible, a sterile needle can be used to remove the spores of the particular mould that is to be isolated.
Isolation of fungi from natural sources is one of the basic skills in mycology that must be mastered by almost everyone concerned with moulds. Isolation techniques are numerous and often complex but can be quite effective in yielding just the mould one wants, while excluding all the others. Isolation techniques can be divided into two broad categories: (1) direct methods and (2) selective methods. Both are routinely used in mycology laboratories and can be further divided into a number of subtypes.
Direct isolation techniques
Direct transfer
The term "direct is applied to techniques involving the simple transfer of a mould from its natural habitat to a pure culture situation in the laboratory. At its simplest this is done by putting a piece of the habitat or substrate under a dissecting microscope so that the mould growth is easily visible. An inoculating needle is then heated until red hot, cooled, and used to transfer some of the spores into a sterile plate of culture medium. I find this easier to do if I first get a tiny piece of agar on the end of the needle for the spores to stick to. The piece of agar must be very small because it is often necessary to manoeuvre it into rather tight places where other moulds might easily contaminate it. An alternative is to moisten the needle with a slight amount of glycerine. Once the transfer has been made, it is only necessary to wait a few days for the mould to grow and form a colony.
The direct transfer technique is also used to obtain pure cultures of mushrooms and other large fungi. The fruiting body is broken open, without touching the newly exposed flesh, and some of the tissue is transferred to a sterile culture medium. If this has been done carefully, the tissue may give rise to a colony after a few days. For the beginner, the easiest large fungi to culture are those found inhabiting decaying wood. Most wood-inhabiting mushrooms and bracket fungi grow well on the semi-synthetic media listed in chapter 4, although few will form their large fruiting bodies in culture.
Moist chambers
Direct isolation of fungi is often more effective if the natural substrate has been kept moist for one to several weeks to allow moulds to grow and sporulate. The easiest method involves a container called a moist chamber (Figure 11). Moist chambers can take any number of forms, but are basically containers holding a material such as cotton, paper, cloth, sterile sand or soil, or peat moss that can be kept moist for several weeks. The specimen is placed on top of the moist material and left until moulds begin to grow on it. In my classes we use glass containers resembling very deep Petri dishes, and like Petri dishes they have loosely fitting lids. For the packing material we nearly always use peat moss, collected in the field and dried for later use. The container is filled with dry moss and then water is added. Peat moss is like a sponge in that it can absorb and hold a tremendous amount of water. When water is added to the moist chamber it soaks up into the moss. The excess can be poured off and the moss squashed down until it forms a moist layer no deeper than about one-quarter of the height of the container. We then place a single or double layer of filter-paper or paper towel over the moss layer so that the specimen does not come in contact with it. Thus prepared, the moist chamber is ready for the specimen.
In many areas peat moss, in its fresh form, is hard to obtain and it is necessary to find a substitute. Although many people use cotton for this purpose, I think that it is best avoided as it is a good substrate for mould growth itself. Instead, try using a layer of fine soil or very fine sand. Soil and sand will not hold water as well as peat, so watch carefully to make sure it does not dry out. But don't make it too wet or all you will get is mud!
When you are ready to add the specimen, moisten it slightly (unless it is already damp) and place it on top of the filter-paper. Cover the dish and leave it in a place where the temperature is reasonably constant.
Within a few days moulds will begin to appear on the specimen. Most beginners are unprepared for the extremely small size of many moulds and tend to overlook them completely. Invariably students using moist chambers for the first time complain that nothing is growing on their specimens, only to have an instructor point out at least half a dozen different moulds! Be sure to examine the material with a magnification of at least 15-20 times and with good bright illumination. Illumination is especially important and should be focused on the area of the specimen that is under examination. When something interesting is found, it can be removed for microscopic examination; but more about that part later.
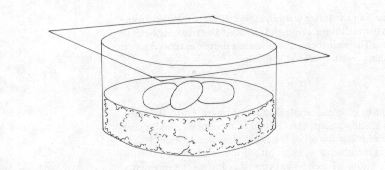
Figure 11. A moist chamber containing three pieces of dung
In order to obtain a pure culture of something in a moist chamber it is only necessary to follow the direct transfer procedure given above, making sure that the sterile needle picks up only the spores that are wanted. If the needle inadvertently touches the surface of the material in the moist chamber it will pick up all sorts of undesirable contaminants.
Moist chambers can be used for all kinds of materials. We have used them to incubate dung, wood, leaves, old stems, corn stalks, bark, seeds, fruits, old fungi, dead insects, and numerous other things. Natural materials are usually more productive than man-made ones, but sometimes old pieces of cloth or leather products can be good. You are only limited by your imagination. Moist chambers are also useful in mycological "detective work", to discover the cause of a particular decay. Placing the decayed material in a moist chamber often will result in abundant sporulation of the guilty organism in the area affected. This technique works well with diseased plant material as well as manufactural products.
With very small specimens, such as insect parts or seeds, it may be easier to use a Petri dish as a moist chamber. We sometimes simply put a few layers of filter-paper in a Petri dish, moisten them, and put the specimen on top. Such moist chambers may dry out very easily, however, and have to be tended closely. A better method is to make up a batch of water agar (a solution of 20 grams of agar in a litre of water), sterilize it, and pour it into sterile Petri dishes. Because the agar solution contains almost no nutrients it will support little mould growth and thus serves only as a water reservoir. The specimen can be placed on the agar surface and examined as in any other moist chamber. Agar media containing nutrients can be used here, but often they only become overrun by the fastest growing moulds at the expense of everything else.
Whether using a moist chamber or working with freshly collected material, one often discovers that moulds produce spores inside some kind of structure, such as a pycnidium. Direct transfer of these structures invariably leads to contamination by other moulds. To avoid the problem it is necessary to clean off the surface of the structure sufficiently that all foreign spores and bacteria are removed. I do this either by pushing the structure over, around, and through an agar medium for 10 minutes or more until it is clean, or by surface sterilizing it for about 60 minutes in a 10 per cent commercial chlorine bleach solution. The required times and concentrations of bleach in the latter technique will differ some what from one specimen to another; the technique is most effective if the treatment can be varied with several samples. The agar technique demands more dexterity but can be applied to a single specimen with predictable results.
Direct plating
Often it is most convenient to place materials that are of interest directly on a nutrient agar medium. As already noted, this technique encourages rapidly spreading moulds at the expense of other fungi but is nevertheless widely used. It is a simple technique, requiring the placing of small bits of the substance on the surface of the agar or the pouring of melted but cooled agar over the fragments. After a few days' incubation mould colonies appear on the surface, and can be transferred into pure culture.
The amount of material placed in the dish varies, depending upon how heavily it is infected with mould spores. This technique is commonly used in soil studies, requiring only a pinch of soil, evenly dispersed over the surface of the agar. It is difficult to apply any rules here, however, because a small amount of material may yield a different set of moulds from a large amount. If the amount of material is large, the result may be a combination of a plating technique and a moist chamber.
We find that Martin's Rose Bengal Medium is a good choice for direct plating, as the rose bengal dye and antibiotics in it slow down colony growth and keep the colonies from growing together at first.
Dilution plating
In this technique 1 gram (dry weight) of the material to be studied is ground up (if necessary) and dispersed in 9 ml of sterile water. One millilitre of this solution is transferred to a second tube containing 9 ml of sterile water, resulting in a 0.01 dilution of the spore mass in the original material. The process is repeated to yield dilutions of 0.001, 0.0001, and 0.00001 or even further if necessary. A 1-ml portion from each dilution is pipetted to a separate Petri dish, and cooled, melted agar medium poured over it. The plate should be moved gently on the table in a figure-of-eight motion to effect proper dispersion. Alternatively, the solution can be put on the surface of solidified medium and spread evenly throughout.
After a few days' incubation, colonies will appear in varying densities, depending upon the amount of dilution from the original material. The number of spores present in the original sample can be calculated roughly by selecting the plates showing 40-100 colonies and writing down the colony count. With this information the following calculation can be performed:

The accuracy of this technique is low when only one plate is counted. There are numerous contributing factors, including improper dispersion of spores during dilution, failure to break up spore masses, or mutual inhibition of growth by certain fungi. Greater accuracy is attained by doing several plates at the most desirable dilution, perhaps ten or more.
Dilutions are frequently used in studies on soil fungi. Although the technique has been criticized as not reflecting the "true" soil flora, it is probably the most commonly used method in this area of study. Even if the fungi isolated are not very representative of the whole flora, the technique does allow scientists to compare one soil with another on a statistical basis.
Airborne fungi
We have already discussed the subject of airborne fungi and have noted that certain moulds are more likely to get their spores into the air than others. Airborne mould spores can serve as an infective agent of plant disease and may also be allergenic. It is well known that allergy sufferers are sensitive to the spores of some moulds but not to others, and it is thus often helpful to know which mould species are present in the air at a particular time or place.
The sampling of airborne fungi is a subject that has attracted much attention in the last twenty years, resulting in the publication of several books. For a thorough treatment of this topic, see Gregory (1973). There are two approaches to air sampling, one that yields spores for microscopic examination and one that yields cultures. The former results in a lot of unidentifiable spores while the latter senses only those that can be cultivated (and many cannot).
It is well beyond my task here to go into all the different sampling techniques that are now in use. We can ignore the spore examination methods completely, as they deal only with spores, and concentrate on the cultural ones. The simplest culture techniques for airborne moulds involve the horizontal or vertical placement of Petri dishes containing a nutrient agar so that they trap any spores that fall on or blow into them. Usually 30 minutes to 3 hours out of doors is required for a good sample using this technique. Indoors the situation is more complicated and depends upon several factors, including the amount of traffic, the type of building, and its cleanliness. A good exposure time for an open Petri dish might be 1-2 hours, but only after testing can one be sure.
It has long been known that open Petri plates and other static spore traps have a rather low efficiency because of the layer of dead air on their surfaces. Any moving air, and its load of spores, will tend to pass over this layer and not reach the surface. To overcome the problem a number of devices have been invented that suck in air and force it against a sticky surface; one device, called an Anderson sampler, accommodates several Petri plates and will even sort spores out according to their size or mass. Such a machine is far more efficient than an open Petri plate but is bulky and is normally used only by specialists.
Baits
Many moulds have quite specific nutrient requirements and are specialized to use materials that other fungi use with difficulty or not at all. We can take advantage of this for the isolation of fungi by presenting a particular substance to the environment for colonization and then later recovering it for isolation of the fungi that occupied it. An example of a fungus sometimes recovered by baiting is the creosote fungus, Amorphotheca resiniae. To isolate it, some scientists coat matchsticks with creosote and place them on soil in Petri dishes for two or three weeks. Amorphotheca, a fungus particularly able to utilize creosote, grows from spores in the soil and invades the matchsticks, where it produces abundant hyphae and conidia. From here it is transferred into pure culture. Man inadvertently baits for this same fungus by allowing small amounts of water condensation to get into tanks containing jet fuel. The fungus grows in the water, utilizing the kerosene for energy, and ultimately forms mats of mycelium that can cause engine failure.
Other kinds of baits might be pieces of wood, insects, carrot chunks, plastics, hair, or anything else one can name. The bait can be submerged in a particular habitat in nature or in a moist chamber. To isolate dermatophytes, for example, it is customary to place hair on moist soil in a moist chamber and examine it periodically for sporulating moulds.
The most commonly baited habitat is water, both fresh and marine. Again, almost anything can serve as a bait and the water can be either naturally occurring or in a Petri dish. Good results can be obtained by putting some pond water in a Petri dish and floating on it a few sesame seeds that have been heated until they have popped. Within three or four days the seeds will be covered with oomycetes producing zoospores. Dead files, pollen, bits of apple or carrot, cellophane, and other materials are also productive baits. Since most moulds attracted this way are true aquatics it is necessary to purify them by transferring them to successive changes of distilled water containing new sesame seeds or other baits. When completely isolated into pure culture they can be transferred to solid media, but they may never sporulate there.
In the natural habitat it is interesting to submerge pieces of wood, whole carrots, apples, etc. on a string for several days or weeks and then bring them in for examination. Submerged wood blocks are one of the most commonly used materials for obtaining marine ascomycetes.
Special techniques
Aside from the rather broadly applicable techniques discussed above, there are many others that are quite specific in their effects. It is well beyond my purpose here to go into even a portion of these techniques, but a few are of particular interest and demonstrate some of the kinds of things that can be done to isolate a new set of moulds from a familiar habitat.
Stress techniques
All moulds are capable of withstanding environmental stresses, but eventually, when the stress is great enough, they will be killed. Not all moulds have the same tolerance to stress, however, and we can take advantage of this property by subjecting a material to just enough stress to kill some moulds but not others. The application of such techniques has turned up another interesting fact: that some moulds will not germinate until they have been subjected to conditions that kill most others. I have already mentioned one such group in my discussion of the dung-inhabiting or coprophilous fungi that produce spores that do not germinate until subjected to the rigours of the digestive tract of animals. Other fungi produce spores that germinate only after they are exposed to fires or freezing.
To explore these interesting adaptations we need only take a sample of soil, dung, wood, etc. and subject it to some kind ot treatment that we kill most fungi. We can steam the substance in an autoclave or steamer (without pressure), soak it in alcohol, acids, bases, or other chemicals, or alternately freeze and thaw it for several weeks. Almost any drastic treatment will yield a few fungal "holdouts" that might not otherwise appear. After treatment, the substance can be handled in any of the normal ways, such as plating, or moist chambers. Dr. De B. Scott (Scott 1968) outlined a method of this type for isolating species of Eupenicillium (an ascomycete) from soil. About 2 grams of soil were added to 18 ml of sterile water and heated in a water bath at 80°C for 30 minutes. After removal from the bath the soil suspension was treated according to the dilution plating technique described above.
Surface sterilization
In the section on direct isolation techniques I mentioned the method of sterilizing the surface of fungi with 10 per cent commercial bleach to kill adhering spores. Surface sterilization can also be used as a selective technique on a slightly larger scale. If we see a living leaf that has a dead circular spot on it we might guess that this is caused by a fungus. If we put the spot directly on the agar medium we will probably get some fast-growing fungus but not the causative agent. What we want is the fungus inside the leaf, not the surface adherents, and a good way to obtain it is to apply bleach long enough to kill the external organisms but not the internal ones. Although materials differ considerably, a sterilization time of about 1 hour in a 10% commercial bleach or 3% hydrogen peroxide will often work. If one is unsure, some longer and shorter times should be tried. Surface sterilization will also work for seeds, large fungi, wood, many man-made materials, dead insects, and other things.
Selective nutrients
This technique is essentially the same as the baiting methods discussed above. It differs only in that the substance to be sampled is added to the "bait" rather than vice versa. We might, for example. wish to isolate those moulds that can utilize cellulose. To select for these fungi we make a medium such as Czapek's, but using cellulose in place of sucrose. If a fungus is to grow well here it must make use of the cellulose; those that cannot will be excluded or grow only very poorly. The possibilities here are almost unlimited, except that when using agar media it is usually necessary to use a "synthetic medium" so that the nutrients that are being modified are known. Because of impurities in the agar, at least some growth of fungi not utilizing the specific ingredient will usually be obtained. These can often be recognized by their sparse growth.
Dr. Barron, in his highly interesting book on eelworm-trapping fungi (Barron 1977), describes a novel medium. He starts a culture of eelworms growing by feeding them dried pea soup and, when they became abundant, adds a little soil. Those fungi that can trap eelworms start to grow and finally dominate the plate. Here the eelworms are the selective nutrient upon which the soil is plated.
There are few organic substances that cannot be metabolized by at least one mould. We can thus prepare media with unusual substances and expect to isolate moulds. Several years ago I made a medium composed of little more than the amino acid tyrosine and isolated a fungus for which I had to describe a new genus and species! The substance one is interested in can be added directly to the medium or a water infusion of a particular substance can be made and agar then dissolved in it.
One commonly used infusion medium is prepared with hay. First 2.5 grams of dry hay are added to 1 litre of water and boiled for 15-20 minutes; then the mixture is strained through cheesecloth, 20 grams of agar are dissolved in the mixture, and it is sterilized as usual. The resulting medium is fairly weak and discourages many fungi that need a rich sugary medium, while encouraging those that are often reluctant to sporulate. Dung infusion agar is prepared in a similar way, but is instead made with 20 grams of dried horse or cow dung. Some materials, such as dead oak leaves or pine needles, will yield an infusion that is toxic to some fungi and not others, resulting in a medium that selects for fungi occupying those materials naturally.
Selective temperatures
Moulds that grow easily at room temperature are said to be mesophilic. Most fungi are mesophilic and are inhibited or killed at unusually high or low temperatures. There are some, however, that actually require unusual temperatures. Moulds requiring high temperatures (40°C or more) are said to be thermophilic, and those requiring low temperatures (15°C or less) are psychrophilic.
In searching for these fungi we simply incubate our plates or moist chambers at the appropriate temperature and isolate the moulds that develop; all isolates and subsequent transfers must be grown at their optimum temperature as well. Bird nests, for example, yield abundant thermophiles when incubated in a moist chamber at 45-50°C, while rabbit dung or compost materials produce several psychrophiles in moist chambers incubated at 0-5°C. The thermophilic forms are amazing for their rapid growth and are, in fact, the fastest growing of all fungi. Some psychrophiles can also grow quickly, however, proving that temperature is not the only factor contributing to rapid growth. Some thermophilic fungi, or at least those that can grow at 37° C, may cause serious infections in man and animals. In working with them, care should be taken to see that they are not exposed to air currents. I do not recommend the isolation of thermophilic fungi in situations involving large groups of students.
Osmophily
Many stored products, such as grain, museum specimens, and hides, undergo degradation by moulds. Most of us have also experienced mould growth on materials stored in damp places at home. Both phenomena are caused by fungi that can withstand unusually dry conditions. Such fungi are said to be osmophilic, a term that refers to their prevalence in environments of high osmotic potential. I have already discussed some of the osmophilic fungi in the section dealing with moulds on foodstuffs and will concentrate here on their isolation.
Osmophiles occur on relatively dry or sugary substances in the home. Most of the fungi encountered on old cloth or leather materials in the basement are osmophiles as are the moulds growing on the surface of jams and jellies. They are abundant enough in the home that they can be isolated simply by opening a Petri plate for 2 or 3 hours in the basement or kitchen. The critical thing is the medium within the Petri plate. Osmophilic fungi grow poorly on normal culture media and often sporulate abnormally or not at all. To isolate them the water actvities of media must be drastically decreased. This means increasing the sucrose in Czapek's Agar from 20 g per litre to 200-500 g per litre, or adding 200-500 g of maltose to each litre in Leonian's Agar instead of the usual 6.25 g. For the identification of osmophilic Aspergillus species, most books will require them to be grown on Czapek's or malt agar with 40% sucrose or on media containing large quantities of glycerine.
Another excellent source of osmophilic fungi is dried fruit. Many producers, however, treat these products with non-toxic fungicides and effectively exclude most moulds. A sampling of dried fruit from several sources may be rewarding, since some may still be productive. As far as is known, the fungi on dried fruits do not produce toxins.
It appears that the seed caches of certain rodents may be good sources of osmophilic fungi, but this habitat has not yet been adequately explored.
Spore printing
Most ascomycetes and basidiomyces and some other moulds are able to eject their spores forcibly. We can take advantage of this property to isolate these fungi by suspending them over an agar surface and allowing the spores to be shot down on it. For mushrooms and bracket fungi a piece of the gill or tube tissue can be attached, perpendicular to the agar, on the lid of a Petri dish. The spores will then float down on to the agar surface and later germinate (if one is lucky). With large mushrooms it is sufficient to attach a single gill, flat side down, to the lid; with small ones the whole cap can be stuck on. We usually use petroleum jelly as an adhesive in our laboratory, but other things, such as masking tape or an agar block, may work as well.
Ascomycetes, such as cup fungi, can also be suspended above an agar surface for spore printing. Some of them, however, are so small that they have to left attached to the material they are growing on and that stuck down as well. The problem of contamination from loose particles dropping down then arises, however, which can be avoided by turning the plate over and letting the spores shoot up. Most ascomycetes can shoot their spores far enough to reach the lid of a Petri dish, but basiomycetes cannot.
Suspending an open Petri dish over a moist chamber can be a good way to trap ascomycetes, particularly from dung. Be sure, though, that the agar does not touch the sides of the chamber of the material within. It is also wise to exclude any air currents that might carry spores upward to the agar.
Interesting isolations can be made from Petri plates that have been opened and inverted over soil, logs, and other materials in the field, if proper care is taken to exclude air currents.
Water agar
Although it is listed and briefly discussed on the pages dealing with culture media water agar is really a quite distinct thing. It can be used in several ways, often with unexpected results. Because it is almost lacking in nutrients it does not allow fungi to grow out luxuriantly as they do on nutrient media. Try one of these techniques:
1. Moist chamber in a Petri dish.
As discussed above, moist chambers allow you to watch a piece of substrate over a period of time to observe the fungi that may appear there. This is usually done with large things like a piece of wood or dung, but it works fairly well for small things too. Water agar will keep a very small piece of substrate in a humid environment for several weeks. Just put the pice of substrate directly on the agar surface and replace the lid. Fucusing on such a tiny subject causes us to use higher magnifications and to observe much smaller fungi. Additionally, organisms in the little piece of substrate may migrate out on to the agar surface and begin to interact with one another. Often the first to appear will be bacteria, followed by swarms of amoebae engulfing them. These may be followed by nematode worms and some fungi, particularly those preying on the nematodes. The action can be quite brisk for a week or two.
2. Effects of nutrient gradient on sporulation.
- Since water agar contains no purpously added substances other than agar, any material placed on it will add some small amount of nutrition. Of course these nutrients will be diluted by the medium as they diffuse out from the material. One technique that takes advantage of this and often seems to work is to cut out a small square of a culture growing on a nutrient medium and place it on water agar. Although the culture may not have produced spores in the rich environment of the original culture it may begin to do so on water agar as its diet becomes more restricted.
3. Observation of mycoparasites.
- Some mycoparasites do not appear to be very effective on fungal hosts growing vigourously on a rich culture medium. If a piece of the host culture, medium and all, is transferred to water agar it will spread out thinly and no longer have it luxuriant growth. If a few spores of the parasite are placed on the thinly spreading mycelium they may be able to germinate and establish a parasitic relationship. We have done this very successfully with species of the mycoparasite Pyxidiophora and its hosts.
4. Marine fungi.
- Some marine fungi, such as species of Paradendryphiella appear to do very well on water agar and sporulate abundantly. This may be due to the trace amounts of marine nutrients in the agar.