Home >> What fungi are >> How fungi reproduce >> Sexual reproduction >> Asci and basidia >> Asci
SEXUAL REPRODUCTION BY MEANS OF ASCI
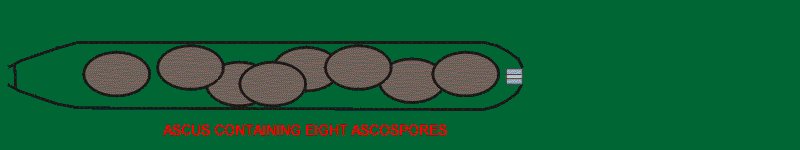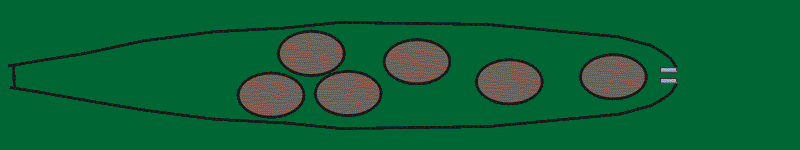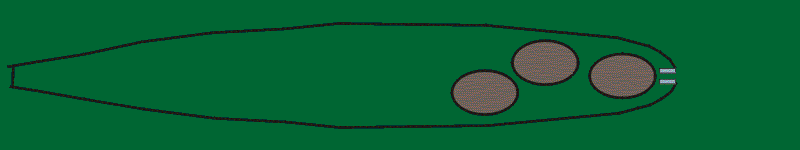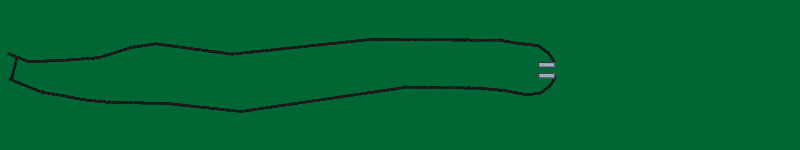
In the Ascomycota the cell in which meiosis occurs becomes modified to form a microscopic sporangium-like structure called an ascus (plural: asci). Usually there is an additional mitotic division to yield eight nuclei. Each of the nuclei becomes surrounded by a cell wall through a process called "free-cell-formation" and develops into a spore called an ascospore. Until the point of free-cell-formation, the animation at right follows the steps outlined in the diagram of meiosis. Compare this with the animation of basidium-formation. While asci and ascospores vary greatly in size, shape, and often colour their function remains constant. Their purpose is to bring about the dispersal and reproduction of the fungus.
Most asci have the ability to shoot their ascospores away from the parent fruiting body. This is accomplished with hydraulic pressure and can be extremely effective. Some of these tiny guns can shoot their spores a distance of several centimeters. An equivalent for a person would be to throw a basketball more than a kilometer. The steps in the discharge of ascospores from an ascus are hard to document because it all happens in a fraction of a second but it appears that the structure at the apex of the ascus is very important. Most asci have a pore or channel at the apex that serves as the exit point for the ascospores. As the ascus reaches maturity it begins to take up water and exert great pressure on its walls. This pressure becomes so great that it finally causes a rupture at the apical pore. When this happens one of the spores is forced up to the pore where it becomes lodged, causing to the pressure to build up again until the spore is shot out like the cork from a champagne bottle. With the release of pressure the next spore becomes lodged in the pore and the process is repeated. After the last spore is discharged the ascus becomes deflated and is no longer functional.
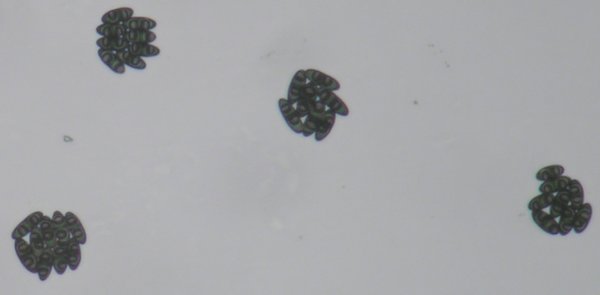
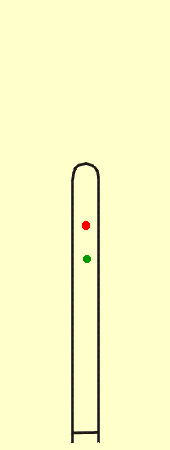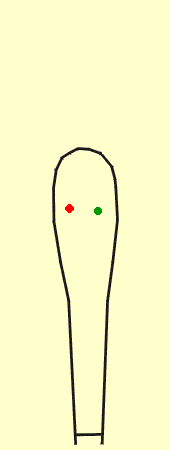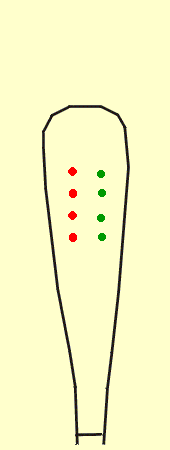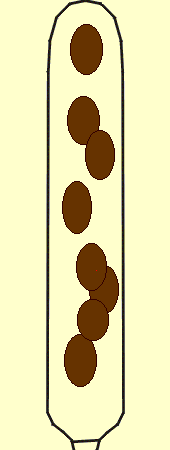
The picture at left shows 32 ascospores. A dead birch branch with perithecia of Melanconis decoraensis, a member of the subclass Sordariomycetidae, was suspended overnight above a microscope slide. In the morning the slide was peppered with groups of eight ascospores, each representing the contents of one ascus. The picture represents the products of four asci.
Asci differ greatly from one another in the structure of their walls and in the makeup of their apical opening. These features are quite constant from individual to individual and reflect the heredity of the species producing them. Because of this "family resemblance", mycologists make great taxonomic use of ascus structure, a subject discussed further in the sections dealing with discomycetes and pyrenomycetes in the Ascomycota.
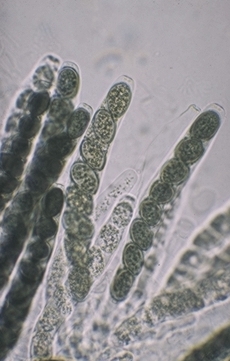
As noted in the previous paragraph most asci are able to forcibly eject their ascospores from the ascus. The illustration of asci of Sordaria fimicola at right are typical of the type that do this. These asci are elongated, more or less cylindrical and contain ascospores suspended in the cytoplasm (cell fluid). Ascospores in some of the asci are darker in colour than those in others, reflecting a slight variation in age. This cluster of asci would have discharged its ascospores over a greater period of time than if all asci matured at the same time. Among the Ascomycota there are species that discharge nealy all of their spores simultaneously while others may stretch dispersal of their spores over many months. This is an intriguing area of natural history that has not had as much attention from mycologists as it deserves.
Ascomycota having forcible ascospore discharge have an efficient means of dispersing their ascospores away from the parent source. This increases the possibility of finding new places to grow and reproduce.
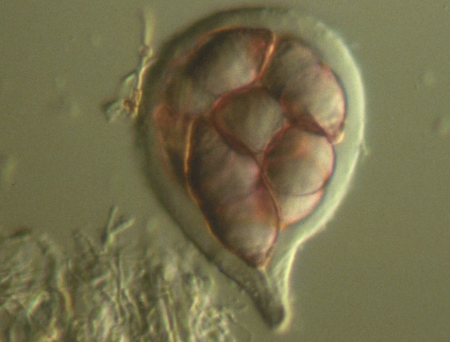
The photographs at left show asci of two species of Ascomycota that do not forcibly discharge their ascospores. The upper picture is of Zopfia rhizophila, a common parasite of asparagus roots. The ascus of Z. rhizophila has a short stalk but is otherwise rounded and contains a packed mass of ascospores. Although the ascal wall is thick it will eventually disintegrate and release the ascospores as a dry mass. The lower of the two pictures shows asci of Aspergillus nidulans, a common inhabitant of rotting compost. Here the asci are even more rudimentary than those of Z.rhizophila and are mainly visible because of the eight red ascospores inside. The two asci at the bottom of the picture are immature and offer a better view of the very thin ascal wall. As with Z. rhizophila, the ascospores of A. nidulans are released to the environment when the ascal wall disintegrates.
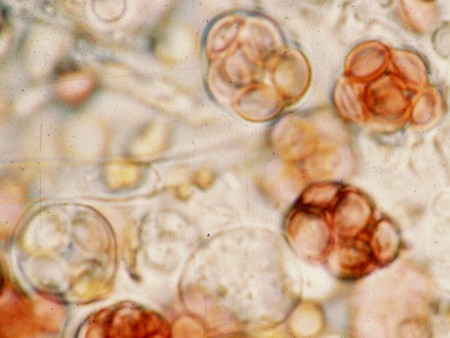
As you might expect, species having evanescent (disintegrating) asci occur in habitats where shooting spores into the air would not be advantageous. Zopfia rhizophila lives underground while A. nidulans is found embedded deeply in its compost; forcibly shot ascospores would simply have nowhere to go. Dispersal away from the parent source must be accomplished by means other than air currents.
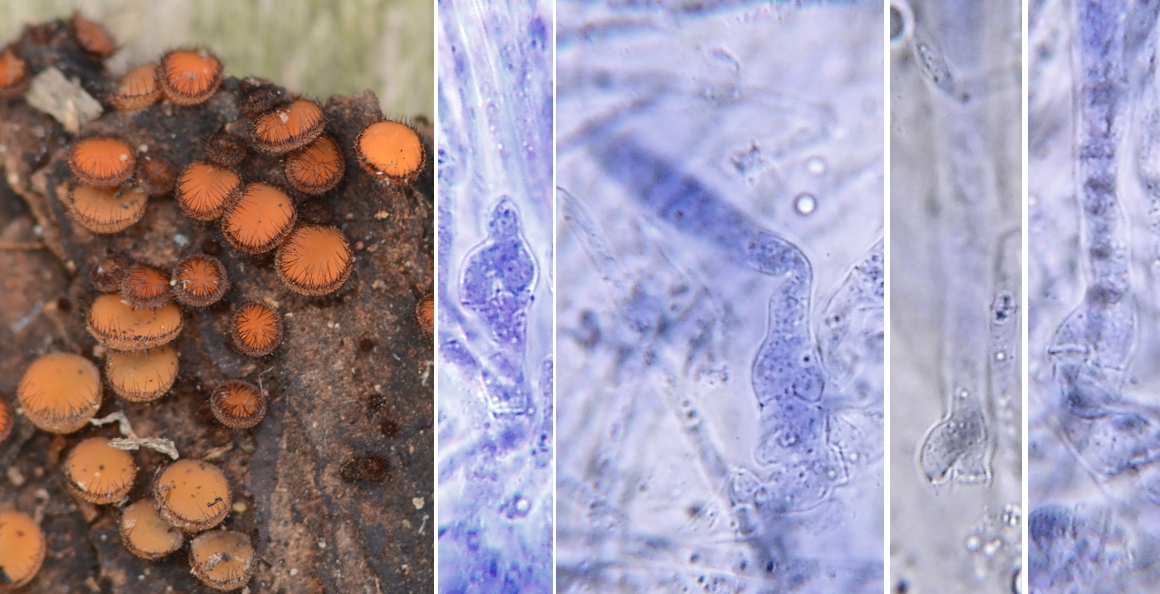
Many asci arise from hook-like structures called croziers. The picture at right shows the common operculate discomycete Scutellinia scutellata with four small panels to illustrate croziers at the base of developing asci. Croziers are notoriously difficult to see with a microscope and often demand patience on the part of the observer. In this case the asci were stained in the dye Ink Blue dissolved in Shear's Mounting medium so that the cell walls could be seen more clearly. A water solution of Congo Red can also be very useful for demonstrating croziers. Nevertheless you may still have difficulty seeing them without further explanation. The drawings at right, although diagrammatic, illustrate how croziers develop.
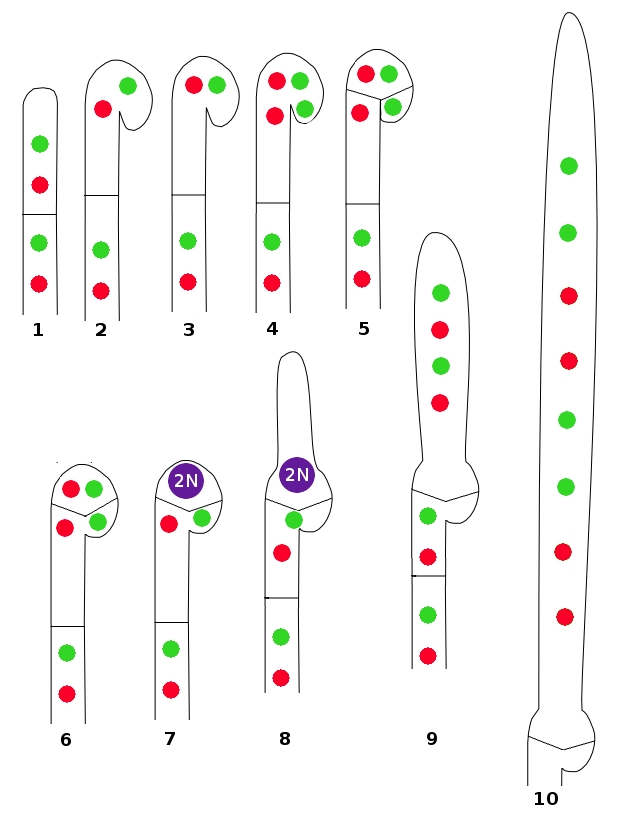
Croziers are quite similar in appearance and function to the clamp connections produced by many Basidiomycota. Their function is to pass a nucleus back to a previously-formed cell, thus maintaining the dikaryotic state. But let's examine the process in more detail using the illustration. Drawing Number 1 shows an undifferentiated hypha with two cells, each containing a pair of compatible nuclei. The hypha begins to curve at the top (2) and nuclei move upward to align themselves side-by-side (3). Each nucleus then divides, temporarily giving the crozier four nuclei (4). Two septa are then formed (5), isolating two nuclei in the top of the crozier and one in the cell behind and one in the end cell. These three cells, from the tip back, are usually referred to as the "ultimate", "penultimate", and "antepenultimate" cells of the crozier. The cell walls separating the ultimate and antepenultimate cells then dissolve, allowing the two nuclei to reestablish the dikaryon (6).
The two nuclei in the penultimate cell fuse to form the diploid (2N) condition (7). At this point the young ascus begins to elongate from the top of the penultimate cell (8). The diploid nucleus undergoes meiosis, leaving the developing ascus with four haploid nuclei (9). There is usually one more cell division to yield eight nuclei, ech of which will become surround by an ascospore wall (10).
Although small and often difficult to demonstrate, croziers are important characteristics used in the identification of many Ascomycota. Their occurrence is sporadic; some species always produce them while others do not. Sometimes two species in the same genus can be separated on their presence and absence. They can be found on all kinds of asci ranging from the large and complex to very simple spherical ones